Auxilium Biotechnologies, a CA-based medical device company focused on remedying peripheral nerve injuries, successfully demonstrated its 3D bioprinting technology on the ISS.
In a first for the ISS and for the nascent field of space-based bioscience, the company was able to fabricate eight medical devices in microgravity within two hours—with minimal input from the astronauts on board.
The mission: Auxilium launched its 3D bioprinter, dubbed the Auxilium Microfabrication Platform, or AMP-1, to the ISS in November.
During the six-week mission, the company demonstrated how its 3D bioprinter can produce a range of medical devices without the burden of gravity.
The bioprinter uses lightweight cartridges filled with a proprietary biological material that can be crafted into a range of implantable devices. The focus initially was to use AMP-1 to fabricate a small implantable medical device to repair peripheral nerve damage.
During production on Earth, gravitational forces lead to an uneven distribution of the necessary particles that promote nerve regeneration. In space, however, microgravity allows for an even distribution, theoretically making the device more effective.
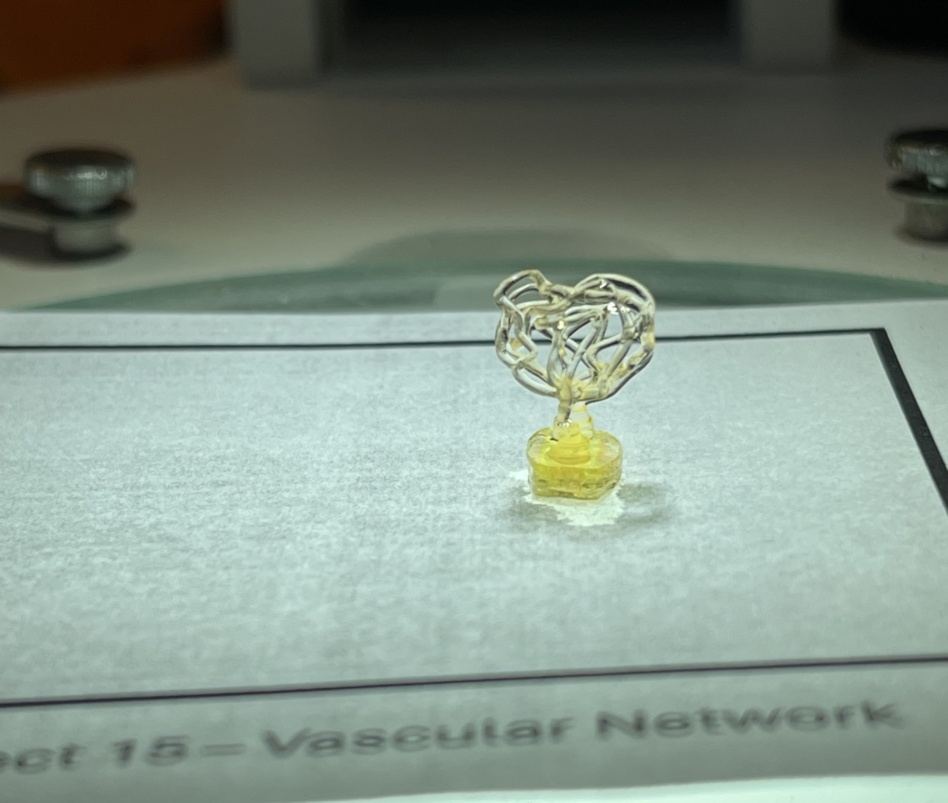
After demonstrating this technology, the company pivoted to see what else they could fabricate. In later trials, the team sent up specs to have the bioprinter craft perfusable vasculature—essentially proving that the system could print blood vessels.
“That wasn’t in the original plan. We just decided we’re going to do it. We designed it. We uploaded this to the printer. We printed the next day,” Auxilium’s CEO Jacob Koffler told Payload.
What’s next: Auxilium now has to overcome the hurdle that is the FDA to begin testing these devices in clinical trials—which is easier said than done.
“The convention is—the dogma is—that space station is not a sterile environment. No one knows what are the risks,” Koffler said. “And of course, the FDA has never seen anything like this before.”
In the meantime, the company has plans to keep printing. The first mission used all of the 20 cartridges of the bioprinter ink, but AMP-1 remains on station ready for future print orders once the cartridges are replenished. Auxilium is in discussions with multiple research labs around the country to collaborate on future projects.
Down the line the company envisions the technology will not only help improve human health back on Earth, but also support medical care for crews on long-duration spaceflight missions.
“We are agnostic to the platform. In that sense, it can be ISS, [or] it can be future commercial space stations. We just plug into the rack, and all we need is power and internet,” Koffler said.